Abstract
Primary ITP is a rare disease defined as thrombocytopenia in the absence of an underlying condition. Characteristic signs include easy bruising, petechial rash and epistaxis. Whilst in many cases the aetiology is unknown, there is a known link with childhood vaccinations.
Objectives: To compare the key features of vaccine associated ITP with non-vaccine associated ITP. Addressing the hypotheses that the severity of bleeding is milder, the clinical course shorter and the requirement for treatment less in vaccine associated ITP.
Methods: Data on 1469 patients was extracted from the UK paediatric registry www.uk-itp.org . The vaccination group included those vaccinated in the 6 weeks prior to the onset of ITP. Their data including; demographics, vaccine type, platelet counts and treatments was then analysed using appropriate statistical methods.
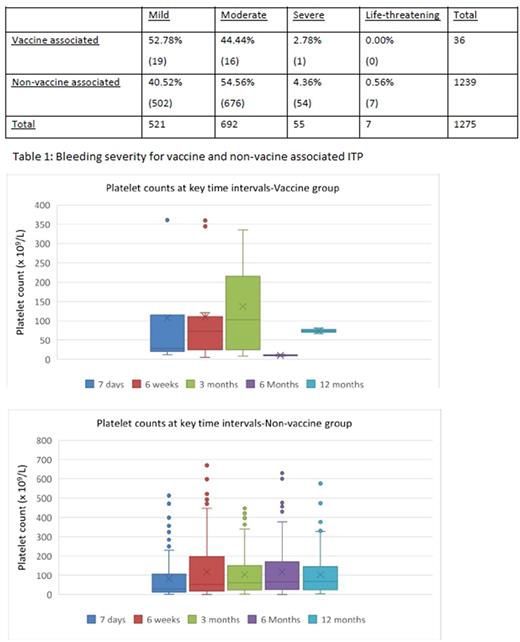
Results: 37 patients had vaccine associated ITP, 51% of which following the MMR vaccine. The median time from vaccination to onset of symptoms was 15 days. 97% of the vaccination group presented with mild or moderate bleeding symptoms compared to 95% of the non-vaccine group. The time for the platelet count to recover was statistically different (P=0.008), being much quicker in the vaccination associated group (median 19 days compared to 99 days in the non-vaccine group). 19% of the vaccine cohort required treatment with either steroids or IVIG which was less than in the non-vaccine group where 21% received treatment (P=0.053).
Conclusion: The data supports the hypothesis that vaccine associated ITP resolves more quickly and requires less treatment than non-vaccination associated ITP.
Grainger: GSK: Honoraria; Amgen Inc.: Honoraria; Novartis: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal